Pioneering new treatment reverses incurable blood cancer in some patients
BBC | 09.12.2025 04:30
A therapy that would once have been considered a feat of science fiction has reversed aggressive and incurable blood cancers in some patients, doctors report.
The treatment involves precisely editing the DNA in white blood cells to transform them into a cancer-fighting "living drug".
The first girl to be treated, whose story we reported in 2022, is still free of the disease and now plans to become a cancer scientist.
Now eight more children and two adults with T-cell acute lymphoblastic leukaemia have been treated, with almost two thirds (64%) of patients in remission.
T-cells are supposed to be the body's guardians - seeking out and destroying threats - but in this form of leukaemia, they grow out of control.
For those on the trial, chemotherapy and bone marrow transplants had failed. Apart from the experimental medicine, the only option left was to make their death more comfortable.
"I really did think that I was going to die and I wouldn't be able to grow up and do all the things that every child deserves to be able to do," says 16-year-old Alyssa Tapley from Leicester.
She was the first person in the world to have the treatment at Great Ormond Street Hospital and is now enjoying life.
The revolutionary treatment three years ago involved wiping out her old immune system and growing a new one. She spent four months in hospital and couldn't see her brother in case he brought in an infection.
But now her cancer is undetectable and she needs only annual check-ups. Alyssa is doing her A-levels, the Duke of Edinburgh Award, eyeing up driving lessons and planning her future.
"I'm looking into doing an apprenticeship in biomedical science, and hopefully one day I'll go into blood cancer research as well," she said.

The team at University College London (UCL) and Great Ormond Street Hospital used a technology called base editing.
Bases are the language of life. The four types of base - adenine (A), cytosine (C), guanine (G) and thymine (T) - are the building blocks of our genetic code. Just as letters in the alphabet spell out words that carry meaning, the billions of bases in our DNA spell out the instruction manual for our body.
Base editing allows scientists to zoom to a precise part of the genetic code and then alter the molecular structure of just one base, converting it from one type to another and rewriting the instruction manual.
Researchers wanted to harness the natural power of healthy T-cells to seek out and destroy threats and turn that against the T-cell acute lymphoblastic leukaemia.
This is a tricky feat. They had to engineer the good T-cells to hunt the bad ones without the treatment annihilating itself.
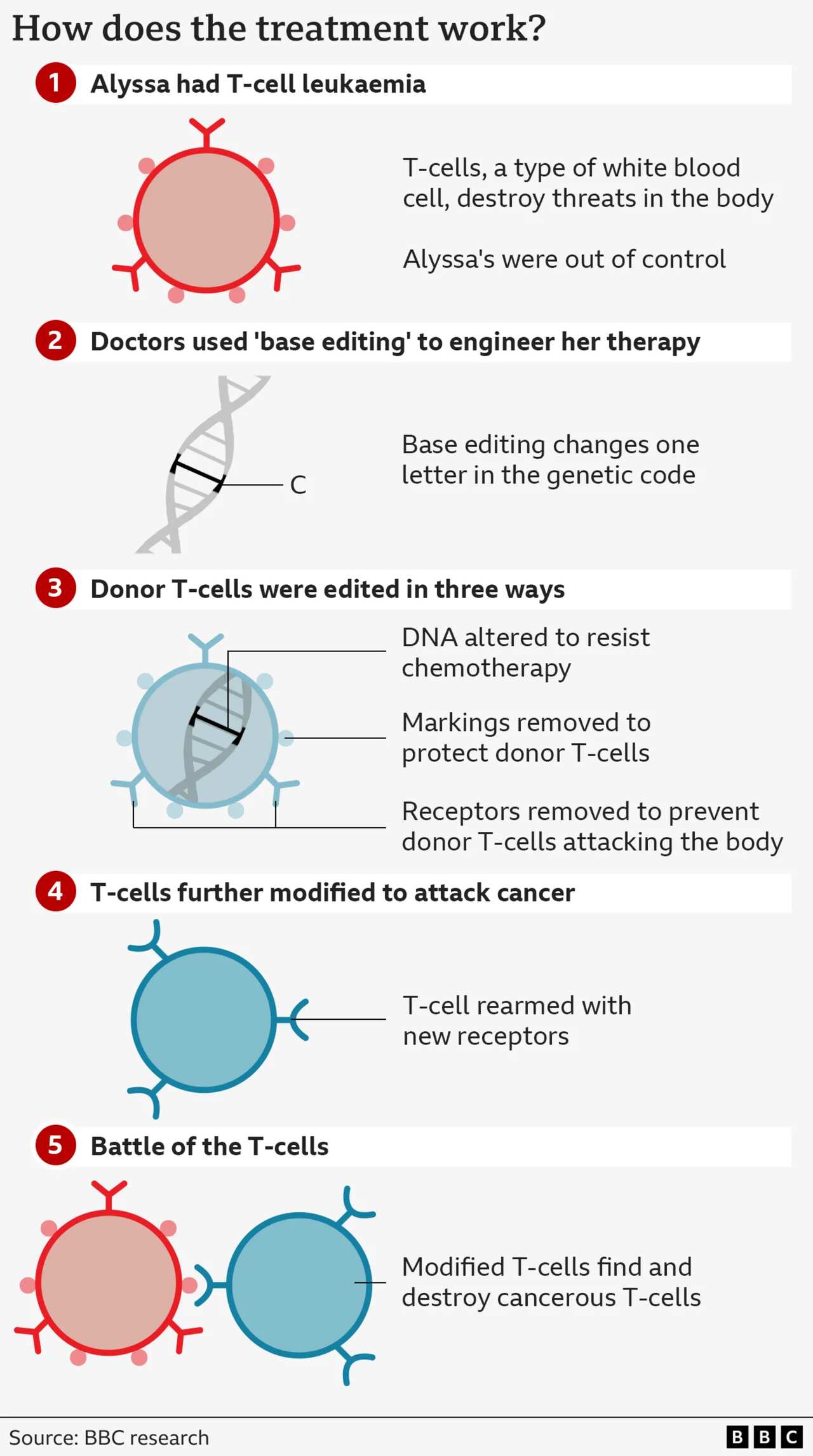
They started with healthy T-cells from a donor and set about modifying them.
The first base edit disabled the T-cells' targeting mechanism so they could not attack the patient's body.
The second removed a chemical marking, called CD7, which is on all T-cells. Removing it is essential for preventing the therapy from self-destructing
The third edit was an "invisibility cloak" that prevented the cells being killed by a chemotherapy drug.
The final stage of genetic modification instructed the T-cells to go hunting for anything with the CD7 marking on it.
Now the modified T-cells would destroy every other T-cell they found whether they were cancerous or healthy, but they would not attack each other.
The therapy is infused into patients and if their cancer cannot be detected after four weeks, then patients have a bone marrow transplant to regrow their immune system.
"A few years ago, this would have been science fiction," says Prof Waseem Qasim from UCL and Great Ormond Street.
"We have to basically dismantle the entire immune system.
"It's a deep, intensive treatment, it's very demanding on the patients, but when it works, it's worked very well."
The study, published in the New England Journal of Medicine, reports the results of the first 11 patients treated across Great Ormond Street and King's College Hospital. It shows nine achieved a deep remission that enabled them to go for a bone marrow transplant.
Seven remain disease-free between three months and three years after treatment.
One of the biggest risks of treatment include infections while the immune system is wiped out.
In two cases, the cancer did lose its CD7 markings, allowing it to hide from the treatment and rebound in the body.
"Given how aggressive this particular form of leukaemia is, these are quite striking clinical results, and obviously, I'm very happy that we managed to offer hope to patients that otherwise have lost it," said Dr Robert Chiesa from the bone-marrow transplant department at Great Ormond Street Hospital.
Dr Deborah Yallop, consultant Haematologist at King's, said: "We've seen impressive responses in clearing leukaemia that seemed incurable - it's a very powerful approach."
Commenting on the research, Dr Tania Dexter, senior medical officer at UK stem cell charity Anthony Nolan, said: "Considering these patients had a low chance of survival before the trial, these results bring hope that treatments like this will continue to advance and become available to more patients."




![[MARCH] Absa RUN YOUR CITY GQEBERHA 10K](https://cdn.thefuse.co.za/images2/8/a/e/a/3/8aea3a79-13e7-34ad-b45d-6fd620f4996a-1173x660.jpg)




